Synapse is an informatics platform bringing together scientific data, analysis tools, and models into a common collaborative research space. In Synapse, registered users can upload, share, analyze and/or export data, analysis codes and results. The principle aim behind Synapse is to promote cooperative data-intensive science. However, in certain cases data sharing must be restricted or closely controlled due to conditions in the informed consent, legal contract and/or privacy requirements. This wiki highlights the rules for contributing, sharing and analyzing data on Synapse.
To learn more about Synapse data use requirements see the links in the sections. If you have questions this wiki does not answer, please contact the Synapse Commons Access and Compliance Team: act@sagebase.org
All Synapse users must have read and agreed to the Synapse Commons Terms and Conditions of Use in order to use the Synapse system
Data in Synapse is assigned to 1 of 3 data access tiers, based on data access restrictions. These are assigned by the Data Contributor and govern data access and distribution by the Data User
Synapse tiers
Open Access
Restricted Access
Controlled Access
See the Synapse Commons Data Sharing Policy for more details
Data Contributor
Rules for Uploading Data to Synapse
Before uploading data to Synapse make sure that all human data is de-identified in compliance with HIPAA guidelines.
For questions about assigning data to any of the 3 access tiers please contact the Synapse Commons Access and Compliance Team: act@sagebase.org
This is data that may be shared with every registered Synapse user in an unrestricted manner
Examples:
- Nonhuman data
- Human non-genotype data without additional limitations
- Human genotype data obtained from:
- Deceased individuals
- Data has already been made public with no restrictions elsewhere
- The individual has consented for data sharing
- Human copy number variation (CNV) data
- Summarized genotype data such as genotype frequencies
This is data that may be shared following user agreement to comply with additional data-specific terms
Examples:
- Limitations on use based on;
- Research field
- Type of analysis
- User affiliation (e.g., nonprofit only)
- Obligation to partner with the data generator
- Requirement for the return of results
This is data that require additional protections for human subjects.
Examples:
- Sequencing and/or genotype data from living individuals
- Data from 'vulnerable populations' as defined by the OHRP
- Identifiable 'special categories' of personal data from European subjects as defined by the European comission (EU Directive 95/46/EC)
Data User
Rules for Accessing & Redistributing Synapse Data
For questions about accessing and redistributing data from any of the 3 access tiers please contact the Synapse Commons Access and Compliance Team: act@sagebase.org
Data labeled as Open Access can be used and redistributed with no other requirements than acceptance of the Synapse Terms and Conditions on Use. All data in Synapse is Open Access unless otherwise specified.
Data labeled as Restricted Access may be used following agreement to comply with additional data-specific terms
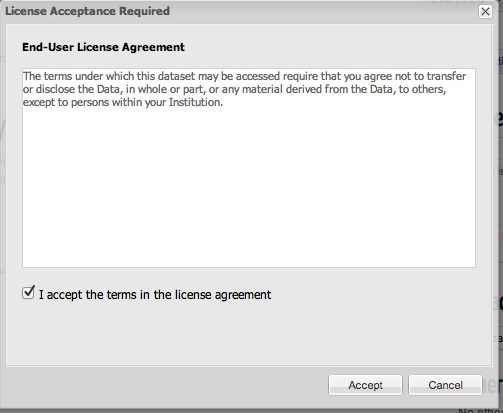
When accessing data that has been assigned the Restricted Access tier you are shown a dialog with the data specific terms of use as created by the data contributor, as shown below. If you accept the terms, then an access approval is created
If you agree to the terms of the Restricted Access data, you are responsible for ensuring that the data is not made openly available by you either through your Synapse projects or in any other way
Data labeled as Controlled Access requires approval for access by the Synapse Commons ACT. This data may not be shared
When accessing data that has been assigned the Controlled Access tier you will be shown a dialog box requiring you to contact the ACT in order to learn the access restrictions specific to these data. This application must contain
- Project Name
- Principal Investigator
- Organization
- Phone Number
- Application Date
- Research Plan
- Non-technical summary
- Listing of Key Personnel
- IRB approval